Enzymes are molecules that regulate the chemical reactions that occur in all living organisms. Almost all enzymes are globular proteins that act as catalysts, substances that speed up chemical reactions. Enzymes catalyze reactions by reducing the activation energy for a specific reaction to occur and yet are neither destroyed nor altered during this process. Understanding how enzymes work in biological systems is a critical and difficult concept for students to comprehend.
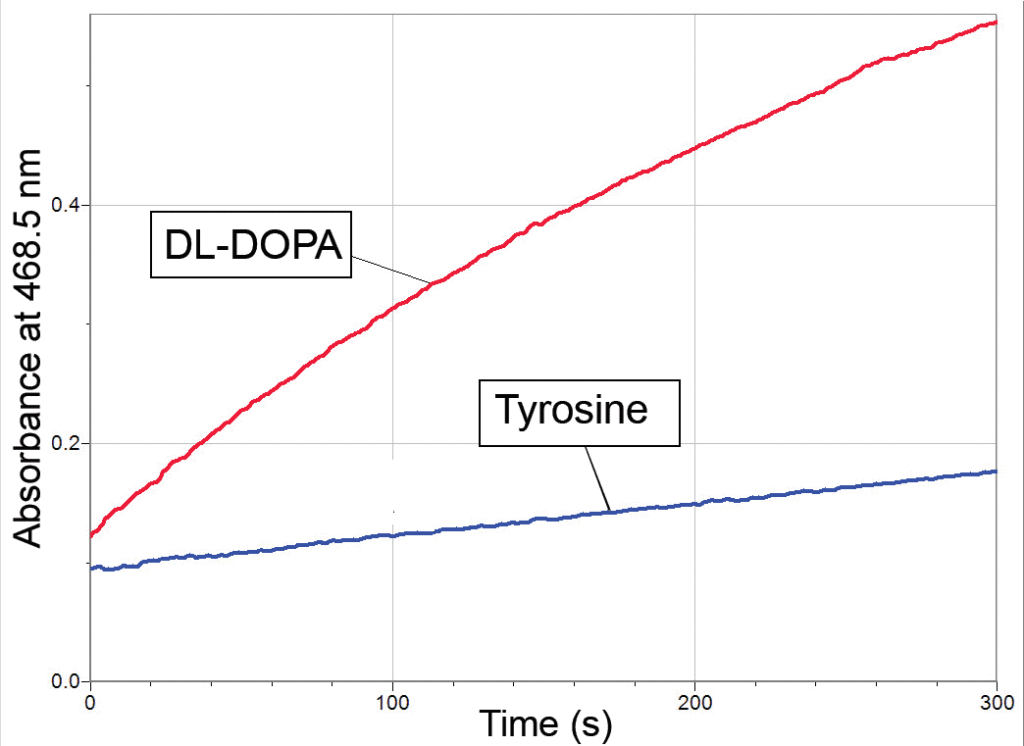
Graph demonstrating that tyrosinase converts DL-DOPA (red) to dopachrome at a much faster than if tyrosine (blue) is used as the substrate
John Melville, our Biology Staff Scientist, developed an advanced laboratory exercise using the Vernier SpectroVis Plus spectrometer and the enzyme tyrosinase. This enzyme is involved in skin coloring in mammals, immunity in insects, and the browning of fruits, tubers, and fungi that have been cut or damaged.
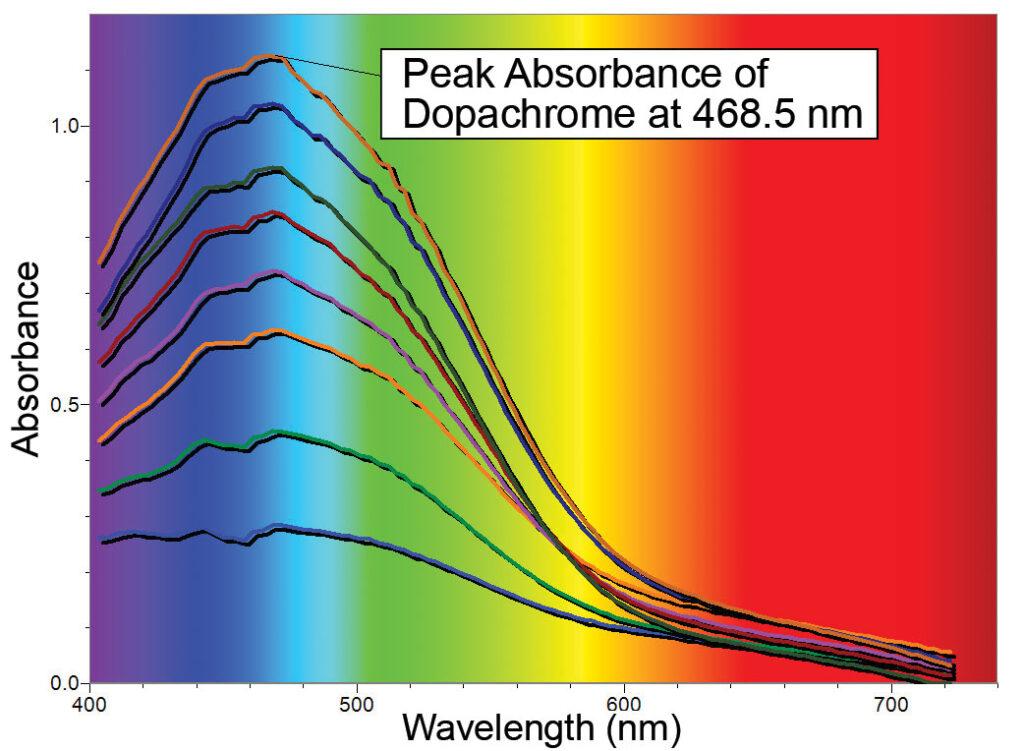
Tyrosinase catalyzes the amino acid tyrosine into the compound DL-3, 4-dihydroxyphenylalanine or DL-DOPA for short. Tyrosinase then catalyzes DL-DOPA into a compound that spontaneously converts into dopachrome. As shown in the first graph, dopachrome is a compound with a peak absorbance at 468.5 nm, and is very easy to detect and quantify using a Vernier SpectroVis spectrometer.
Tyrosinase is an enzyme that lends itself to the student laboratory. The crystal structure of this enzyme from sweet potato has been published (see Klabunde et al., 1998). Enough enzyme for an entire class period can be isolated from a single potato, and the enzymatic conversion of DL-DOPA to dopachrome can be measured in as little as 2 minutes. In addition, many different compounds act as inhibitors and alternative substrates for this enzyme. For example, the conversion of tyrosine to DL-DOPA is the rate-limiting step in this series of reactions. This is clearly shown in the sample data provided in the second graph above, where DL-DOPA is converted to dopachrome at a much faster rate than when tyrosine is used as the substrate.
In the first part of this advanced laboratory exercise, students prepare extracts of the enzyme tyrosinase from potatoes. They then compare the reaction rates of two different substrates—tyrosine and DL-DOPA. Students then investigate the role of increasing substrate concentration on enzyme activity. The second part of the lab is a guided inquiry exercise that involves quantitative analysis and the role of inhibitors, pH, or temperature on tyrosinase.
Download Tyrosinase Enzyme Activity »
This lab is experiment #15 in the Advanced Biology with Vernier lab book, which includes essential teacher information. The Logger Pro file for this experiment is included in the latest software update.
