Experiments
Here are experiments our science specialists have selected to support the IB* topic.
Identifying an Unknown Analgesic by Three Methods
Experiment #4 from Organic Chemistry with Vernier
In this experiment, you will
- Calculated the Rf values of acetylsalicylic acid, acetaminophen, caffeine, and ibuprofen.
- Determine the melting temperature of each of these analgesics.
- Identify the solvent system for good separation.
- Use TLC and melting temperature to identify your unknown analgesic.
- Use absorbance spectroscopy to further identify your unknown analgesic.
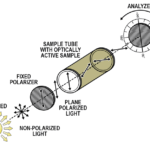
Understanding Polarimetry
Experiment #6 from Organic Chemistry with Vernier
In this experiment, you will
- Become familiar with the use of the Polarimeter.
- Experience how sample path length and concentration affect observed rotation.
- Calculate the specific rotation for a known sugar sample using Biot’s law.

Investigating Gas Chromatography
Experiment #8 from Organic Chemistry with Vernier
In this experiment, you will
- Measure and analyze the chromatogram of a mixture of five compounds as they pass through a Vernier Mini GC.
- Vary the temperature-pressure profile of the Mini GC and observe how the chromatogram is affected by such changes.
- Determine the best temperature-pressure profile to obtain clear separation of all five compounds.
Investigating Thermodynamic Relationships of Substituted Hydrocarbons
Experiment #11 from Organic Chemistry with Vernier
In this experiment, you will
- Collect and analyze GC data from various samples.
- Calculate the equilibrium constant between the various compounds and the GC column.
- Use the equilibrium constant to identify the change in free energy of solution.
- From the temperature dependent data, calculate the change in enthalpy and entropy of the solution.
- Observe the changes in Gibb’s free energy with the number of carbon atoms in the samples studied.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Additional Higher Level (AHL)
- Topic
- 21. Measurement and Analysis
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
