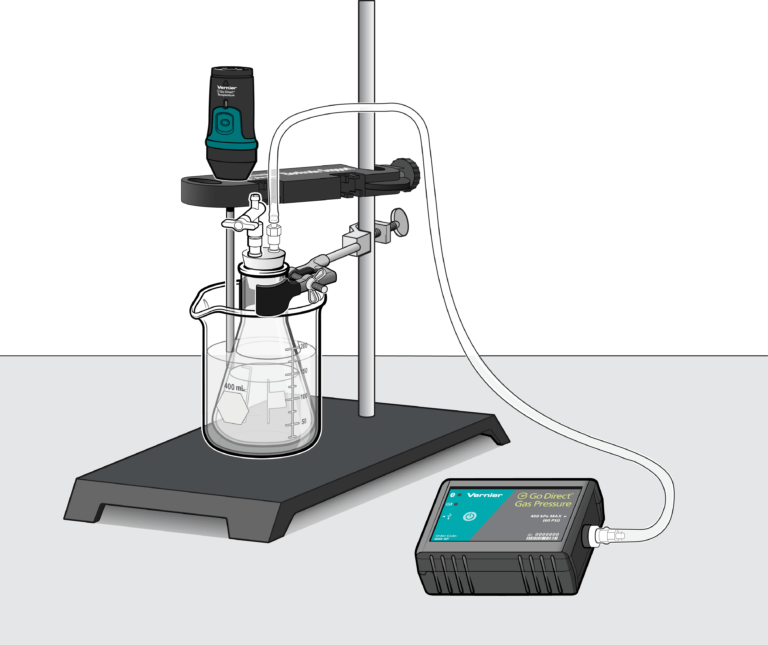
Introduction
We think of a gas as a collection of tiny particles in random, thermal motion. When they collide with the sides of a container, they exert a force on the container walls. The average force resulting from these collisions on each unit area of the container is called the pressure exerted by the gas. You are familiar with everyday units of pressure, such as psi (pounds per square inch) to describe tire pressure or inches of mercury to describe atmospheric pressure. For this experiment, we will use the SI unit of pressure, the pascal, which is defined as one Newton of force acting on each square meter of surface. Since the Newton is smaller than a pound and a square meter is much larger than a square inch, we will use kilopascals, kPa, to describe the pressure of a gas.
You will certainly recognize what variables might affect the pressure of a gas in a container. In this experiment, you will develop quantitative relationships between pressure and these variables.
Objectives
In this experiment, you will
- Collect pressure vs. volume, pressure vs. number, and pressure vs. temperature data for a sample of air in an enclosed container.
- Determine relationships between these pairs of variables.
- Determine a single expression relating these variables.
- Determine the constant of proportionality for the relationship between pressure, volume, and temperature.
- Use kinetic molecular theory (KMT) to model the behavior of the gas at various points on each graph.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Physics
- The students should understand that ideal gases are described in terms of the kinetic theory and constitute a modelled system used to approximate the behaviour of real gases
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #1 of Advanced Physics with Vernier — Beyond Mechanics. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





