Arson Analysis: Using Gas Chromatography
Experiment #15 from Forensic Chemistry Experiments
- Subject
- Chemistry
Introduction
It is not unusual for accelerants used in arson cases to be mixtures of flammable compounds. Your job will be to identify the components of an accelerant recovered by crime scene techs from the scene of an arson.
All types of chromatography are used to separate mixtures. Many forensic tests involve chromatography. There are many different types of chromatography: paper, thin layer, liquid, high-pressure liquid (HPLC) and gas (GC). Chromatography is applied in many fields. Biochemists use liquid chromatography to separate proteins; chemists use GC and HPLC to identify organic compounds. Technicians use GC for drug tests, toxin screens and environmental analysis.
All chromatography methods operate under the same principles. There is a stationary phase and a mobile phase. The mobile phase travels along the stationary phase (in the column or on a plate) from a start point to an end point. Compounds can travel from start to finish at different rates, depending on whether they tend to “stick” to the stationary phase or “float” in the mobile phase. Compounds stick to the stationary phase through dipole interactions, dispersion forces or ionic interactions.
The Mini GC uses a metal column, the inside of which is coated with the stationary phase. A sample, consisting of one or more compounds, is injected onto the column and is pushed through by air, which acts as the mobile phase. Organic compounds flowing out of the chromatography column are seen as a peak on a chromatograph, as seen in Figure 1. The amount of time it takes for a compound to exit the column after it is injected is called the retention time. With a GC, a compound can be identified from a mixture of chemicals by its retention time.
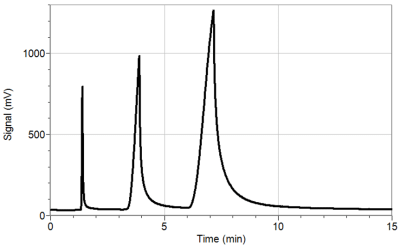
Figure 1 Sample gas chromatogram
Several factors can affect the interaction of a compound with the GC. More volatile compounds (i.e., compounds with a lower boiling point) tend to move through the column faster because they are flowing in the mobile phase and interacting very little with the stationary phase. The functional groups present on the compound are also a factor. For example, alcohols may interact with a polar stationary phase more than esters because alcohols can form stronger hydrogen bonds. The molecular weight of a compound can also play a role, although it is not a simple matter of saying that the heavier the molecule, the slower it will travel through a GC column.
Objectives
- Create gas chromatograms for crime scene accelerant and known compounds.
- Identify the components of the accelerant collected from the crime scene.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Structure 2.2.10—Chromatography is a technique used to separate the components of a mixture based on their relative attractions involving intermolecular forces to mobile
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #15 of Forensic Chemistry Experiments. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.