Introduction
A majority of the world’s total energy comes from the burning of fossil fuels. Coal, natural gas, and petroleum (which includes oil and gasoline) are all used extensively throughout the world. The energy content of a fossil fuel is an important property. This property helps scientists and engineers determine the usefulness of a fuel. Energy content is the amount of heat produced by the burning of 1 gram of a substance, and is measured in units of joules per gram (J/g).
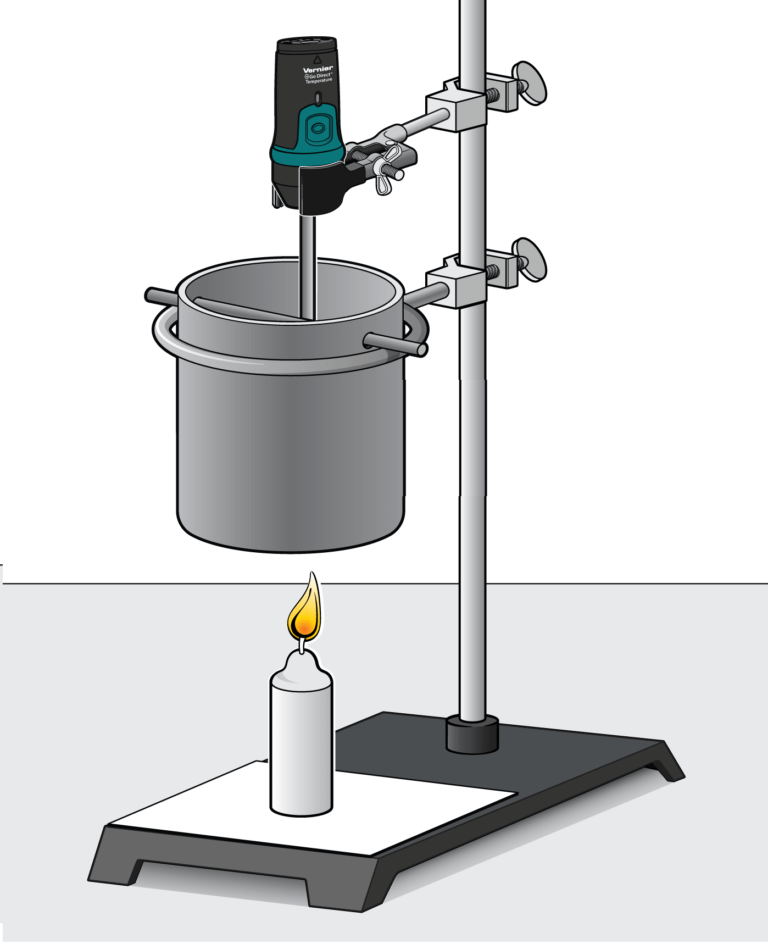
In this experiment, you will determine the energy content of a fuel by burning an amount of the fuel and capturing the heat released in a known mass of water. You will use a temperature probe to measure the initial and final temperatures of the water, and then calculate the energy released.
Objectives
- Use a temperature probe to measure the temperature of water.
- Use a balance.
- Determine the energy content of fuels.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #30 of Earth Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.



