Determining Avogadro’s Number
Experiment #31 from Advanced Chemistry with Vernier
- Education Level
- High School
Introduction
The basic counting unit in chemistry, the mole, has a special name, Avogadro’s number, in honor of the Italian scientist Amadeo Avogadro (1776-1856). The commonly accepted definition of Avogadro’s number is the number of atoms in exactly 12 g of the isotope 12C, and the quantity itself is 6.02214199 × 1023.
A bit of information about Avogadro seems appropriate. His full name was Lorenzo Romano Amedeo Carlo Avogadro (almost a mole of letters in his name). He was a practicing lawyer until 1806 when he began his new career teaching physics and math at the University of Turin, where he was later promoted to the chair of physical chemistry. In 1811, Avogadro published a paper in the Journal de Physique, entitled “Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds,” which pretty much says it all. This paper includes the statement that has come to be regarded as Avogadro’s Hypothesis:
The first hypothesis to present itself in this connection, and apparently even the only admissible one, is the supposition that the number of integral molecules in any gases is always the same for equal volumes, or always proportional to the volumes. Indeed, if we were to suppose that the number of molecules contained in a given volume were different for different gases, it would scarcely be possible to conceive that the law regulating the distance of molecules could give in all cases relations as simple as those which the facts just detailed compel us to acknowledge between the volumes and the number of molecules.
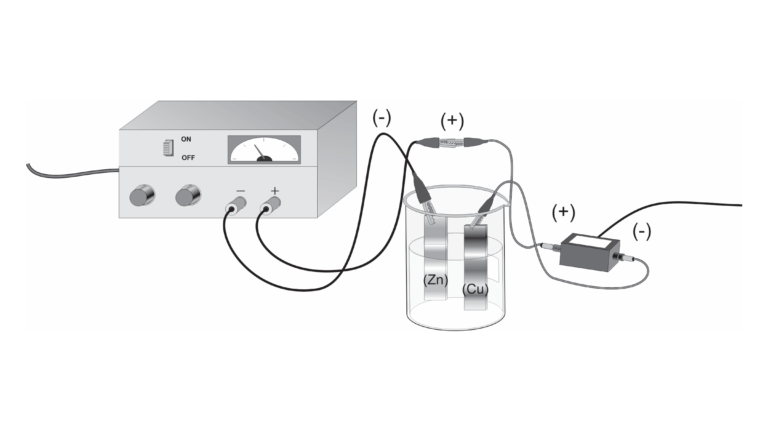
In this experiment, you will confirm Avogadro’s number by conducting an electrochemical process called electrolysis. In electrolysis, an external power supply is used to drive an otherwise nonspontaneous reaction. You will use a copper strip and a zinc strip as the electrodes, placed in a beaker of sulfuric acid. You will make the cell electrolytic by using the copper strip as the anode and the zinc strip as the cathode. By determining the average current used in the reaction, along with the knowledge that all of the copper ions formed are the 2+ cations, you will calculate the number of atoms in one mole of copper and compare this value with Avogadro’s number.
Objectives
In this experiment, you will
- Prepare an electrochemical cell to oxidize a copper electrode.
- Measure the amount of copper that was deposited in the electroplating process and determine the average current used.
- Calculate a value for Avogadro’s number and compare it to the accepted value.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 2

Option 3

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Structure 1.4.1—The mole (mol) is the SI unit of amount of substance. One mole contains exactly the number of elementary entities given by the Avogadro constant.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #31 of Advanced Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


