Experiments
Here are experiments our science specialists have selected to support this IB* topic.
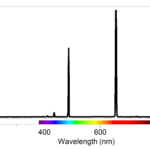
Spectrum of Atomic Hydrogen
Experiment #21 from Advanced Physics with Vernier — Beyond Mechanics
In this experiment, you will
- Use a spectrometer to determine the wavelengths of the emission lines in the visible spectrum of excited hydrogen gas.
- Determine the energies of the photons corresponding to each of these wavelengths.
- Use a modified version of Balmer’s equation to relate the photons’ energies to specific transitions between energy levels.
- Use your data and the values for the electron transitions to determine a value for Rydberg’s constant for hydrogen.
- Educational Standard
- International Baccalaureate (IB) 2025
- Subject
- Physics
- Theme
- E Nuclear and quantum physics
- Topic
- E.1 Structure of the atom
- Level
- Standard level and higher level
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
