Experiments
Here are experiments our science specialists have selected to support the IB* topic.
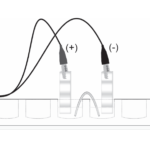
Electrochemistry: Voltaic Cells
Experiment #20 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare a Cu-Pb voltaic cell and measure its potential.
- Test two voltaic cells that use unknown metal electrodes and identify the metals.
- Prepare a copper concentration cell and measure its potential.
- Prepare a lead concentration cell and measure its potential.
- Use the Nernst equation to calculate the Ksp of PbI2.
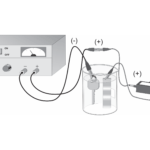
Electroplating
Experiment #21 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare and operate an electrochemical cell to plate copper onto a brass surface.
- Measure the amount of copper that was deposited in the electroplating process.
- Calculate the amount of energy used to complete the electroplating process.
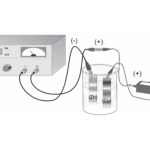
Determining Avogadro's Number
Experiment #31 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare an electrochemical cell to oxidize a copper electrode.
- Measure the amount of copper that was deposited in the electroplating process and determine the average current used.
- Calculate a value for Avogadro’s number and compare it to the accepted value.
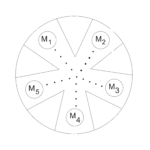
Establishing a Table of Reduction Potentials: Micro-Voltaic Cells
Experiment #28 from Chemistry with Vernier
In this experiment, you will establish the reduction potentials of five unknown metals relative to arbitrarily chosen metal.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Core
- Topic
- 9. Redox Processes
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
