Experiments
Here are experiments our science specialists have selected to support the IB* topic.
The Determination of an Equilibrium Constant
Experiment #10 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare and test standard solutions of FeSCN2+ in equilibrium.
- Test solutions of SCN− of unknown molar concentration.
- Determine the molar concentrations of the ions present in an equilibrium system.
- Determine the value of the equilibrium constant, Keq, for the reaction.
Separation and Qualitative Analysis of Cations
Experiment #14A from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare and analyze a solution that contains ten selected cations.
- Analyze an unknown solution that contains a selection of cations.
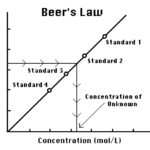
Determining the Concentration of a Solution: Beer's Law
Experiment #17 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare and test the absorbance of five standard copper (II) sulfate solutions.
- Calculate a standard curve from the test results of the standard solutions.
- Test the absorbance of a copper (II) sulfate solution of unknown molar concentration.
- Calculate the molar concentration of the unknown CuSO4 solution.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Additional Higher Level (AHL)
- Topic
- 13. The Periodic Table—The Transition Metals
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
