
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

At my school Archbishop Hoban High School in Akron, Ohio, I teach a semester of organic chemistry as an advanced science elective. Students who choose this course learn about carbon-based compounds, from alkanes to arenes, and use a range of Vernier instruments, from melt stations to mini gas chromatographs. Taking this class not only gives my students more opportunities to hone their lab skills, but it also helps them to develop their understanding of organic compound behavior and physical properties of matter.
One key concept we explore in this class is the melting points of organic compounds. This lesson can easily fit within a chemistry unit on covalent compounds or intermolecular forces—it’s also a valuable lesson to include in AP chemistry courses when covering the properties of substances and mixtures (Unit 3). Investigating this physical property can help students understand how materials are characterized by measurable properties, how molecular forces influence those properties, and why materials with different properties are better suited to different practical uses. In particular, melting points are useful for making predictions about the purity of a substance and about the identification of an unknown compound.
Making Real-World Connections
One easy way to make this concept relevant to students is by connecting it to real-world applications or phenomena, such as baking and food chemistry. For example, you can start the lesson by examining the melting point of table sugar (sucrose), which is 160° C or 320° F and is used in candy-making or baking. At 160° C, the solid sugar melts into a clear liquid, but at higher temperatures will become a darker brown liquid—or caramel. Likewise, different fats used in baking vary in melting points. Butter or margarine may not be the best fat to use in baking since the melting point is around 38° C or 100° F, whereas vegetable shortening melts at 45° C or 111° F. Bakers may choose vegetable shortening in their recipes since it melts at a higher temperature and gives the same flaky texture as butter or margarine. Table salt (sodium chloride), on the other hand, has a much higher melting point of 801° C because of its strong ionic bonds.
Explaining Molecular Forces
Understanding the strength of melting point can be tricky for some students, so I like to use an intrapersonal and interpersonal skills model to help introduce this idea to my students. I explain that if students have strong intrapersonal skills, they have a close relationship with themselves (I demonstrate this by hugging myself). Thus, chemical compounds with robust intramolecular forces have a close relationship—or shorter bond lengths. On the other hand, if students have strong interpersonal skills, they more easily stay connected with others under strain (I ask two students to stand up with me and hold hands with each other while pulling in opposite directions). Therefore, chemical compounds with sturdy intermolecular forces can handle tension, hold together with other molecules like themselves, and melt at a higher temperature. This analogy helps students connect abstract chemical concepts to familiar experiences—this one works well for me, but I encourage you to find connections that resonate with your students!
From Solid to Liquid: The Melting Point Experiment
In this experiment, my students work to identify an unknown organic compound by comparing it to a set of known pure compounds (like benzoic acid or dextrose). They use Vernier Go Direct® Melt Stations to observe how each compound melts at specific temperatures, watching the phase changes through the Melt Station’s wide-angle magnification window. As they collect data using Graphical Analysis on their iPads, they can easily capture screenshots of their data and graphs and integrate them into lab reports later on.
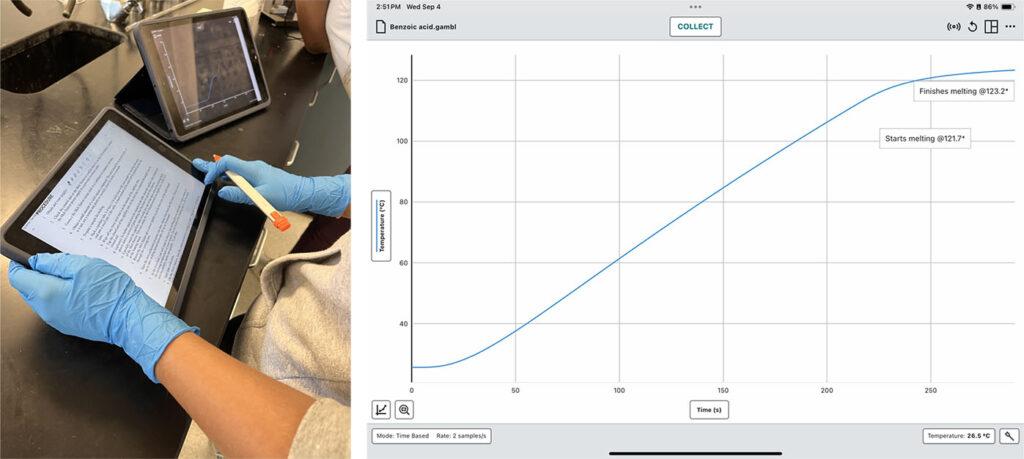
I recommend having students measure the melting point twice for each compound since they use both their physical observation skills (i.e., seeing the change from solid to liquid) and real-time data collection in Graphical Analysis concurrently—which is a good reason to put students in pairs for this investigation. One of the biggest challenges that students may face is pinpointing the exact temperature range when a solid fully melts due to the dynamic equilibrium that exists during a thermodynamic process. To address this, I make sure we discuss what to expect before starting the experiment, including the 2–3°C melting point range, and again, ensure students are in groups or pairs so that they each can observe the melting process and observe the data recorded on the iPad®. It may take multiple tries to find the correct melting point range and replicate their results, but this process helps them actively develop critical thinking, observation, and data-collection skills.

From Liquid to Solid: The Recrystallization Experiment
After students have measured the melting points of pure compounds, we move on to recrystallize an impure compound, such as benzoic acid mixed with copper(II) sulfate pentahydrate. This is a great opportunity to revisit the core chemistry idea that each pure substance has characteristic physical and chemical properties. We talk about how the impurities in the benzoic acid weaken the intermolecular forces—much like a brick wall that has been interspersed with defective bricks. Observing the recrystallization of an impure compound gives students a clear, hands-on demonstration of how impurities impact physical properties.
For those teaching AP, organic, or general chemistry, incorporating melting point investigations with Vernier’s Go Direct Melt Station is a great way to tie together concepts like intermolecular forces and physical properties. Encourage your students to engage actively by repeating measurements and troubleshooting discrepancies in their data. These experiments not only reinforce their understanding of key chemistry concepts but also help them build practical lab skills in analyzing and interpreting data and developing models that will serve them well in more advanced coursework.
| HS-PS1-3 | ||
| Science and Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyzing and interpreting data | PS1.A Structure and Properties of Matter | Patterns |
| Using mathematics and computational thinking | Structure and Function | |

Diane teaches chemistry at Archbishop Hoban High School in Akron, Ohio, and is a member of the Vernier Trendsetters Community. She recently earned her doctorate of education in curriculum and instruction and is passionate about making STEM education more accessible and engaging for all students.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






