
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

Get ready for National Chemistry Week with Vernier! This year, the American Chemical Society (ACS) is celebrating the chemistry behind photography and imaging. From traditional film cameras to cutting-edge medical imaging technologies, chemistry plays a critical role in capturing, creating, and revealing the visuals that shape how we experience the world.
Join us in celebrating National Chemistry Week with three experiments from Vernier that explore the science behind light and color, fluorescence, and radiological images.
1. Reflection and Absorption of Light
Experiment #23 from Earth Science with Vernier

All cameras work by capturing light and recording it onto a medium. In traditional photography, light waves enter the camera and cause a chemical reaction on light-sensitive film. In digital cameras, light enters the camera and hits a sensor that converts light into an electrical signal. But light is not just a technical element—it’s also essential to the creative process in photography, influencing how we perceive color!
A white object appears white because it reflects nearly all wavelengths of visible light that shine on it, whereas a black object absorbs most of the visible light spectrum, reflecting very little back to our eyes. A sunflower, for example, reflects some wavelengths and absorbs others, giving it its characteristic yellow, brown, and green hues. By understanding the reflection and absorption of light, aspiring photographers gain more control over technical precision and creative expression in their images.
In this experiment, students investigate how different colors reflect light and how energy absorption affects temperature. Using a Go Direct® Light and Color Sensor, students measure light reflection and calculate the percent reflectivity of different colored pieces of paper. Then using a Go Direct Temperature Probe, they measure temperature changes caused by energy absorption.
Objectives
- Use a light sensor to measure the amount of reflected light.
- Calculate percent reflectivity of various colored paper.
- Use a temperature probe to estimate the amount of energy absorbed by the paper.
2. Secret Message
Experiment #10 from Forensic Chemistry Experiments
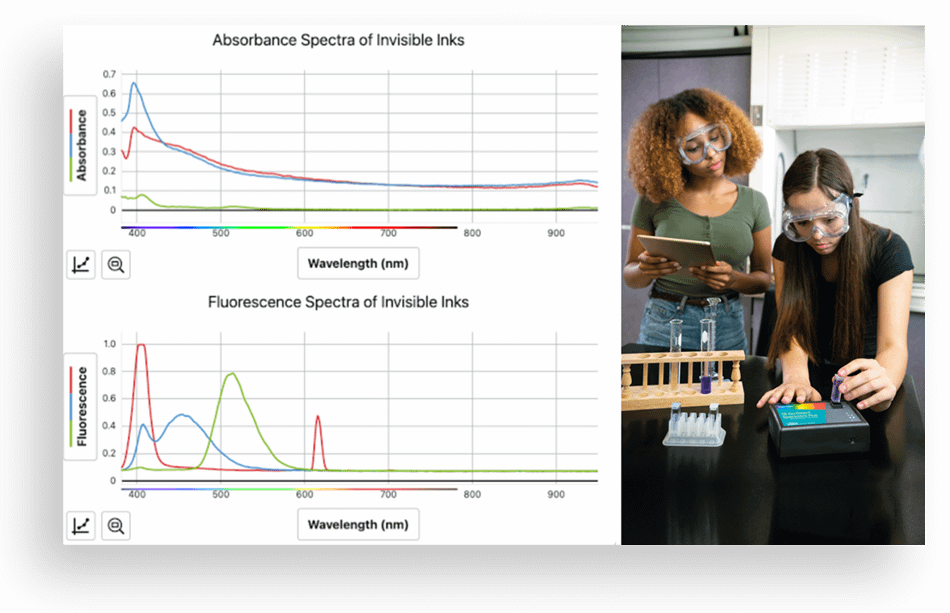
Fluorescence has the power to reveal hidden images, like fingerprints, bloodstains, invisible ink, and even concealed artwork! This makes it a valuable tool in fields ranging from forensic science to medical imaging.
The process of fluorescence occurs when certain chemicals absorb energy from light at one wavelength (or color), causing them to become excited and reach a higher energy level. As these excited states are often unstable, the chemical will re-emit some light at a different wavelength, allowing it to return to a lower energy state.
In this experiment, students gain experience creating and analyzing fluorescence spectra using invisible ink from markers that fluoresce red, blue, and green. Using a Go Direct SpectroVis® Plus Spectrophotometer, they observe and measure wavelengths to calculate frequency and photon energy and use these characteristics to identify different solutions.
Objectives
- Capture absorbance and fluorescence spectra of invisible inks in solution.
- Analyze the spectra to identify the wavelengths of maximum absorbance and fluorescence for each ink.
- Use mathematical representations to calculate photon frequency and energy.
- Interpret data to explain the difference in energy of the photons producing the spectra for each ink.
3. Radiation: α, β, and γ
Experiment #27 from Advanced Chemistry with Vernier
Modern medical imaging technologies like X-rays, CT scans, and MRIs rely on different kinds of light and sound waves to create images of the human body. Nuclear medicine, however, uses radioactive tracers to diagnose and treat disease. These tracers emit gamma rays, which specialized imaging devices can detect, allowing doctors to study internal organs and tissues in real time. This type of imaging is often used to detect cancer, monitor heart conditions, and evaluate organ function by tracking how tracers move through the body.
Nuclear radiation is broadly classified into three categories, labeled with the first three letters of the Greek alphabet: α (alpha), β (beta), and γ (gamma). Alpha radiation consists of fast-moving helium nuclei, which are relatively heavy and carry two positive charges. Beta radiation consists of electrons or positrons, which are much lighter and carry one unit of charge. Gamma radiation consists of photons, which have no mass and no charge, but carry more energy than X-rays.
After being emitted from a decaying nucleus, alpha, beta, or gamma radiation may pass through matter, or it may be absorbed by the matter. In this experiment, students use the Go Direct Radiation Monitor to test how air, paper, and aluminum interact with each type of radiation by using small sources of alpha, beta, and gamma radiation.
Objectives
- Develop a model for the relative absorption of alpha, beta, and gamma radiation by matter.
- Use a radiation counter to measure the absorption of alpha, beta, and gamma radiation by air, paper, and aluminum.
- Analyze the count rate data to test for consistency with your model.
Looking for more chemistry inspiration? Check out our recent Mole Day webinar!
Take a look at these additional resources from ACS to engage your students with the chemistry behind photography and imaging! Plus, explore our full range of chemistry solutions.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






