
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM
Vernier offers more than 1,000 experiments in biology, chemistry, engineering/robotics, and physics that can help you inspire students and integrate data-collection technology into your STEM classes.
All three of this month’s featured experiments involve our spectrometers. To further explore the use of spectrometers, check out our Illuminate Spectroscopy webinar.
Biology
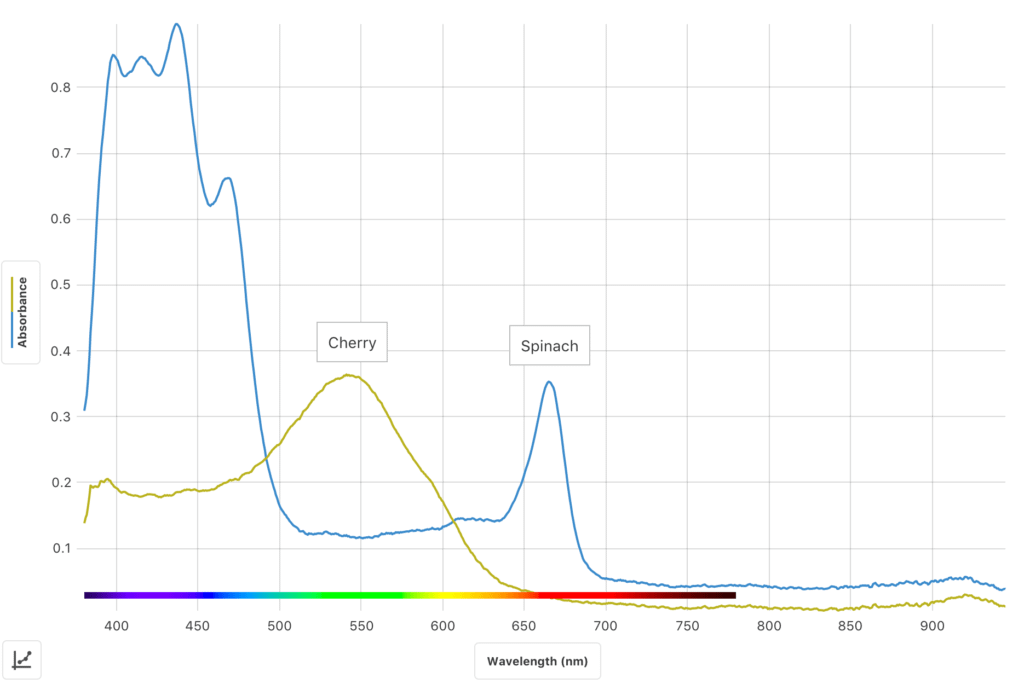
In this inquiry-based experiment, students use the Go Direct® SpectroVis® Plus Spectrophotometer or Go Direct Fluorescence/UV-VIS Spectrophotometer to
- Determine and analyze the visible light absorbance spectrum of spinach extract.
- Determine and analyze the fluorescence spectrum of the same spinach extract sample.
- Develop and investigate a researchable question dealing with visible light spectra and/or fluorescence spectra of plant pigments.
Chemistry
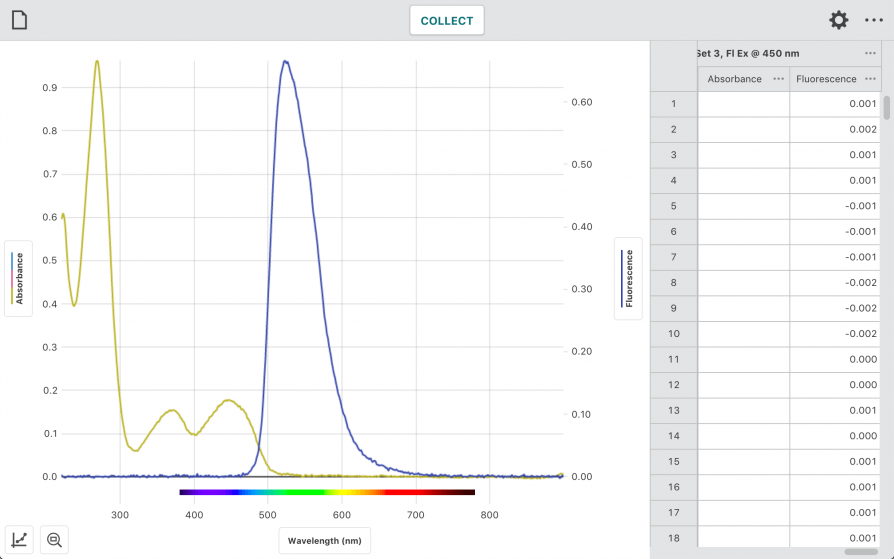
“Why Does Your Energy Drink Glow?”
In this experiment, students use the Go Direct SpectroVis Plus Spectrophotometer or Go Direct Fluorescence/UV-VIS Spectrophotometer to
- Measure the absorbance spectrum of riboflavin to determine the best wavelength for fluorescence excitation.
- Observe fluorescence of vitamin B2 in energy drinks.
- Measure a fluorescence calibration curve of vitamin B2 standards.
- Calculate the concentration of vitamin B2 in energy drinks.
- Compare measurements to stated concentration to determine accuracy.
Physics
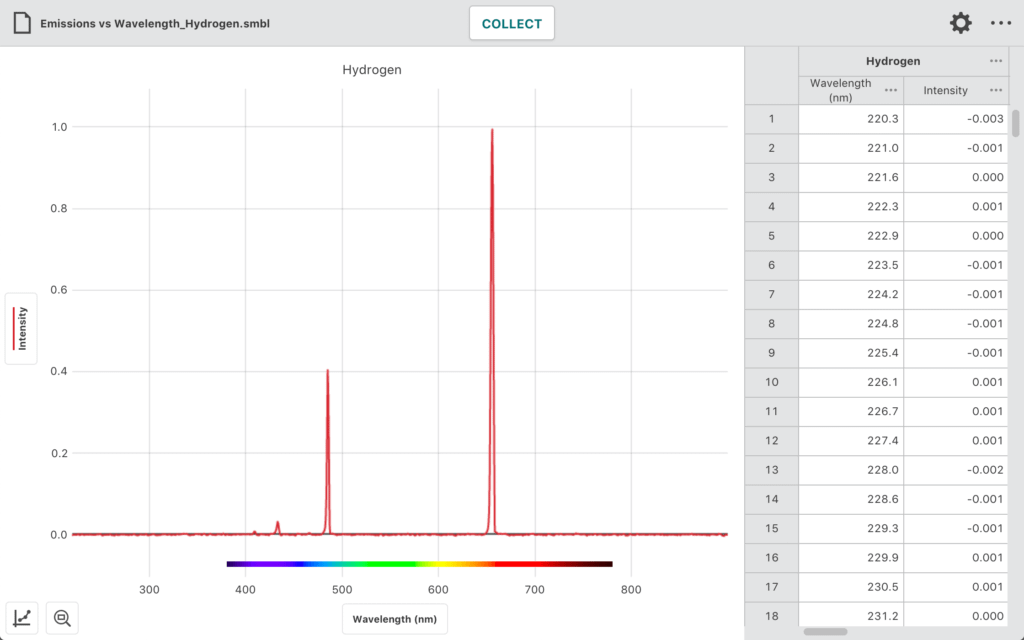
In this experiment, students use the Go Direct Emissions Spectrometer and other equipment to
- Determine the wavelengths of the emission lines in the visible spectrum of excited hydrogen gas.
- Determine the energies of the photons corresponding to each of these wavelengths.
- Use a modified version of Balmer’s equation to relate the photons’ energies to specific transitions between energy levels.
- Use their data and the values for the electron transitions to determine a value for Rydberg’s constant for hydrogen.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






