
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

Every year, the College Board releases a Chief Reader Report that gives a behind-the-scenes look at how students did on the AP Chemistry free-response questions. It highlights the most common misconceptions, errors, and sticking points—and it’s a goldmine for teachers. Along with sample student responses and scoring guidelines, it’s one of the most useful tools AP chem teachers have for understanding where students are struggling and how we can better support them.
I taught chemistry and physics in Maryland for 34 years and also spent time as an AP exam scorer, so I know how valuable this kind of insight can be. Reading through the report (and yes, I really do read it!) helps me think about how we’re preparing students—not just for the test, but for deeper understanding.
To dig into this year’s results, I teamed up with Dr. Melissa Hill, our Director of Chemistry at Vernier to look at two of the lower-scoring free-response questions on the 2024 AP Chem Exam. In this first post, I’m focusing on Free-Response Question 1, which tackled titrations and calorimetry. Below, I’ll walk through some of the big takeaways from the Chief Reader Report, point out where students had the most trouble, and share Vernier experiments and strategies that can help reinforce these core concepts.
Free Response Question 1: Lactic Acid and Sodium Hydroxide Titration/Calorimetry
Topics Covered: Molecular Structure of Acids, Acid-Base Titrations, pH and pKa, Solutions, Calorimetry, Enthalpy of Reaction*
Max Score: 10
Mean Student Score: 4.43
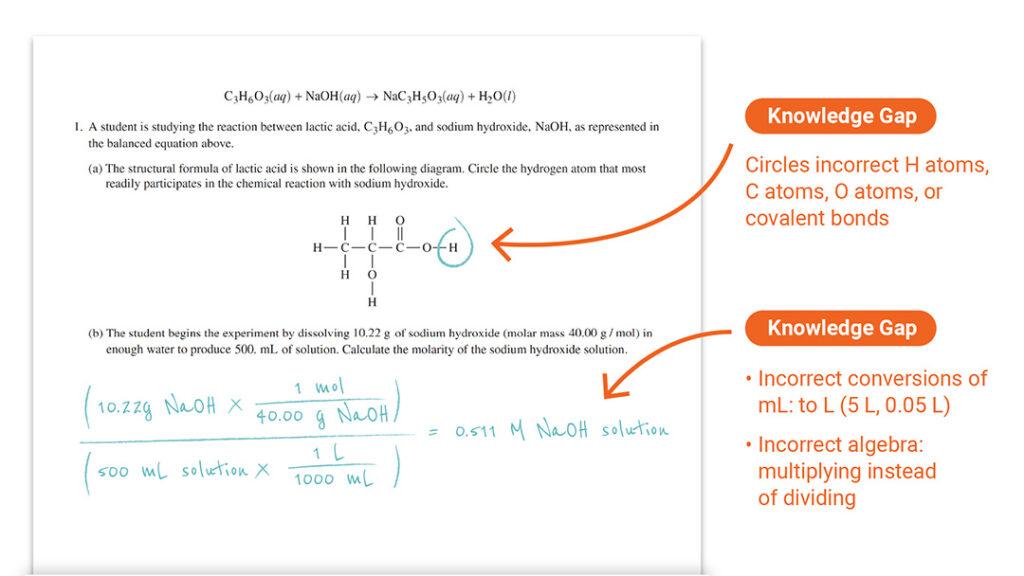
Question 1 Part (a): Structure of Acids and (b): Calculating Molarity
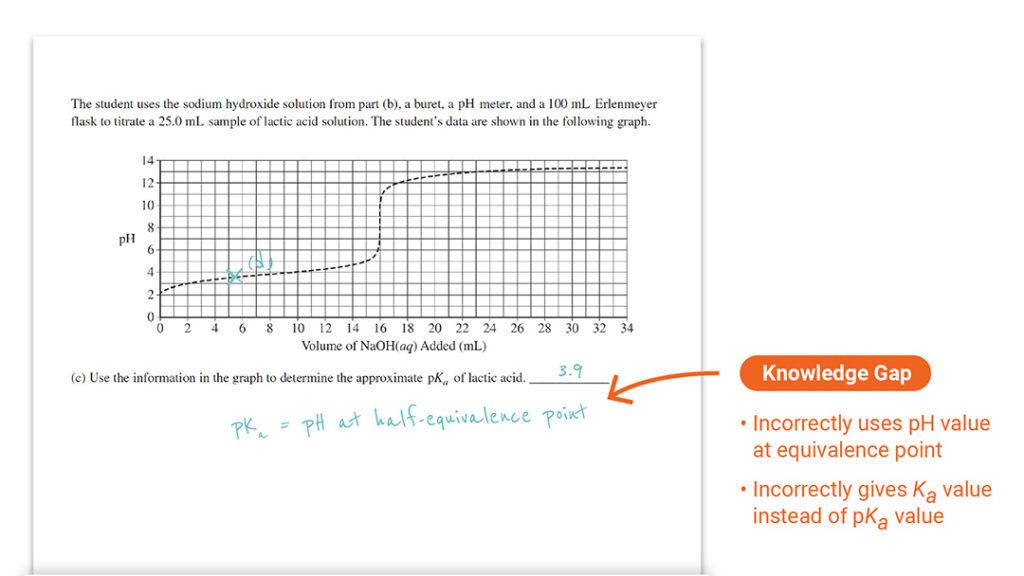
Question 1 Part (c): Identifying pKa
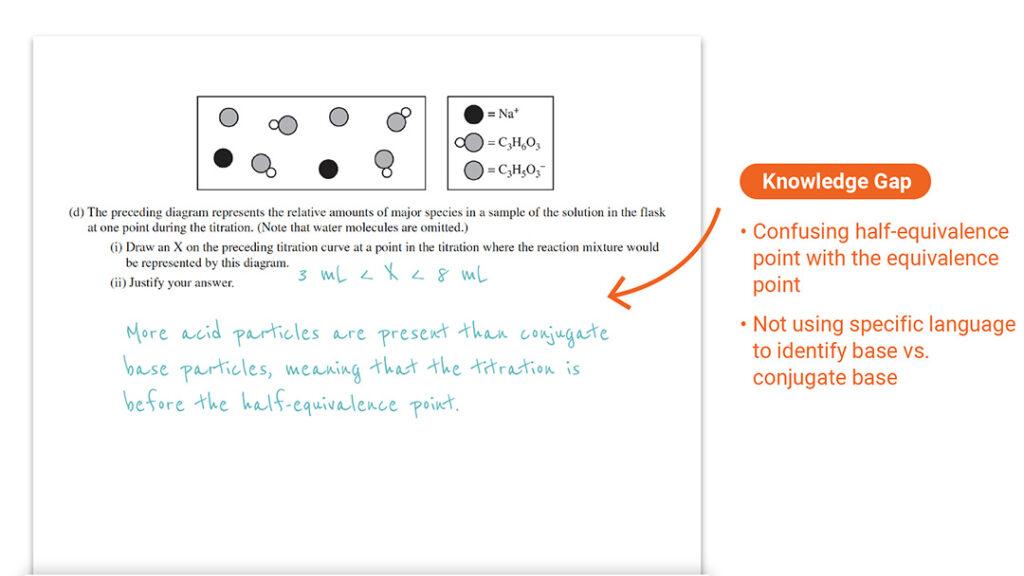
Question 1 Part (d)(i) and (d)(ii): Understanding Half-Equivalence vs. Equivalence Point
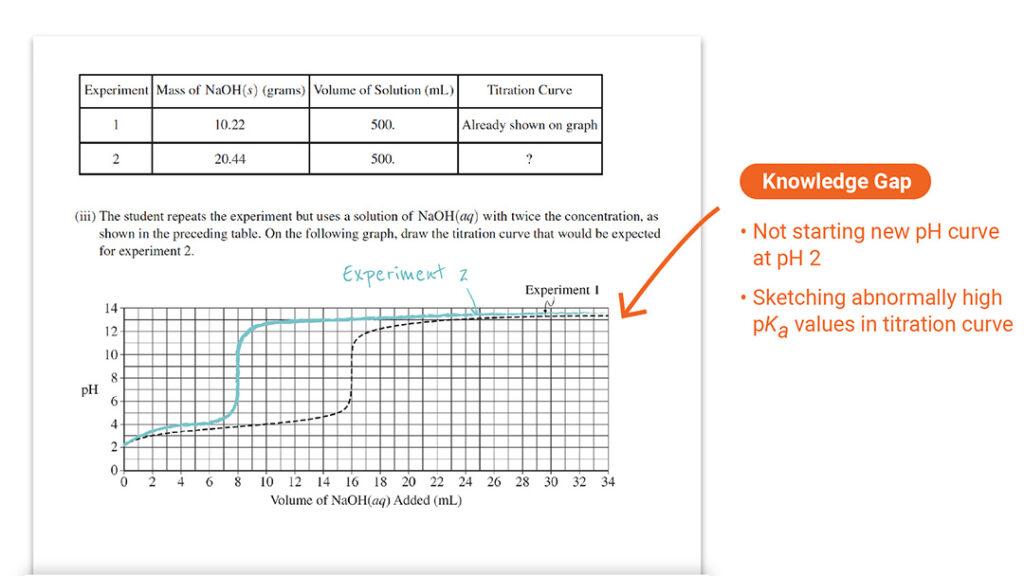
Question 1 Part (d)(iii): Sketching the Titration Curve

Question 1 Part (e)(i): Calculating the Quantity of Heat Produced

Question 1 Part (e)(ii): Calculating the Molar Enthalpy

Question 1 Part (e)(iii): Justifying Your Conclusion
Question 1: Key Takeaways
After digging into the patterns of student mistakes on Question 1, the Chief Reader Report offers some really helpful guidance for us in the classroom. And honestly? I think they’re spot on. Here are a few recommendations that stood out to me—and ones I’ve found make a real difference when working with students:
- Hands-on Lab Experience Matters: Students tend to do better on experiment-based questions when they’ve actually done the experiments in the lab. If acid-base titrations and calorimetry experiments haven’t made it into your rotation yet, it’s worth finding time for them.
- Predicting Experimental Outcomes: One of my favorite ways to deepen understanding is to have students consider how a small change in the setup would affect the results. You can even have lab groups run slightly different versions of the same experiment and compare their findings—it really gets them thinking.
- Interpreting Particulate Diagrams: Students should feel comfortable identifying what’s happening in a titration based on particulate representations—especially at these key points:
- Before the half-equivalence point
- At the half-equivalence point
- After the half-equivalence point
- At the equivalence point
- After the equivalence point
Try These Experiments in Your Lab
Determining the Enthalpy of a Chemical Reaction with a Temperature Probe
Students use a temperature probe to collect data as they dissolve different salts in water, calculate molar enthalpies of reaction, and analyze heat flow. This experiment directly supports the thermochemistry reasoning required in Question 1 and helps students interpret their results in context.
This calorimetry experiment has students burn food samples and calculate the energy released in kilojoules per gram per serving, helping reinforce the math and meaning behind enthalpy and heat flow. It’s great for giving students real-world applications while strengthening their data analysis skills.
This experiment gives students a hands-on, observable way to connect temperature changes to energy transfer in chemical reactions. It reinforces the concept that enthalpy change (ΔH) reflects the heat exchanged with the surroundings and shows how multiple reactions can be combined using Hess’s law to determine the ΔH of a reaction that can’t be measured directly.
Acid-Base Titration with a pH Sensor

In this experiment, students conduct titrations, record pH and volume data, and generate titration curves. They identify equivalence points to determine the acid concentration and calculate the pKa and the equilibrium constant (Ka) to identify it—all directly relevant to parts (c), (d)(ii), and (d)(iii) of Question 1.
This experiment compares a strong acid (HCl) and a weak acid (acetic acid) to help students visualize how titration curves differ. It’s a great reinforcement tool for understanding how pKa relates to the shape of the graph and what’s happening at different stages of the titration.
A student favorite with a fun forensic twist—students perform a titration to figure out the concentration of NaOH in a makeup remover. They use the pH curve to find the equivalence point and do follow-up calculations, just like they’re asked to do on the AP exam. Check out our webinar on this experiment.
Looking for More AP Chemistry Support?
Whether you’re starting a new AP program or looking for new ways to reinforce tricky AP topics, Vernier has you covered. Check out these resources for more webinars, teacher tips, and classroom-ready investigations aligned with the AP Chemistry framework.
- Vernier Chemistry Investigations for Use with AP Chemistry: Our AP-aligned lab book includes 16 guided-inquiry and hands-on experiments that help students build conceptual understanding through data collection and analysis.
- AP Chemistry Purchasing Guide: This helpful overview covers recommended sensors and equipment for a complete AP Chemistry lab setup.
- Top Three Topics That Students Miss in AP Chemistry Prep—and How to Tackle Them: This blog post focuses on common student struggles and practical ways to support them with targeted experiments.
- From Sparks to Spectacular Data: Exploring Hess’s Law and Combustion: This webinar explores introducing students to calorimetry, enthalpy, and molar heats of reaction through a demonstration of burning magnesium and temperature probe investigations.
- Chemical Clues: A Titrating Tale of Makeup Remover Contamination: This webinar uses real-world forensic applications to engage students and explore key AP concepts like pH curves, equivalence points, and concentration calculations.
- Past AP Chemistry Exam Questions: Download free-response questions from past exams along with scoring guidelines, sample responses from exam takers, and scoring distributions.
*All exam questions, knowledge gap summaries, and takeaway commentary are based on the 2024 AP Chemistry Chief Reader Report and supporting materials provided by the College Board.
**AP and Advanced Placement Program are registered trademarks of the College Entrance Examination Board, which was not involved in the production of and does not endorse this product.
We’re here to help! Reach out to chemistry@vernier.com, call 888-837-6437, or drop us a line in the live chat.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






