pH is a quantitative unit of measure that describes the degree of acidity or alkalinity of a substance. It is measured on a scale of 0 to 14. The formal definition of pH is the negative logarithm of the hydrogen ion concentration (i.e., pH = –log10[H+]). In practice, it is the hydrogen ion activity that is measured, rather than its concentration. The activity is a measure of the “effective concentration”.
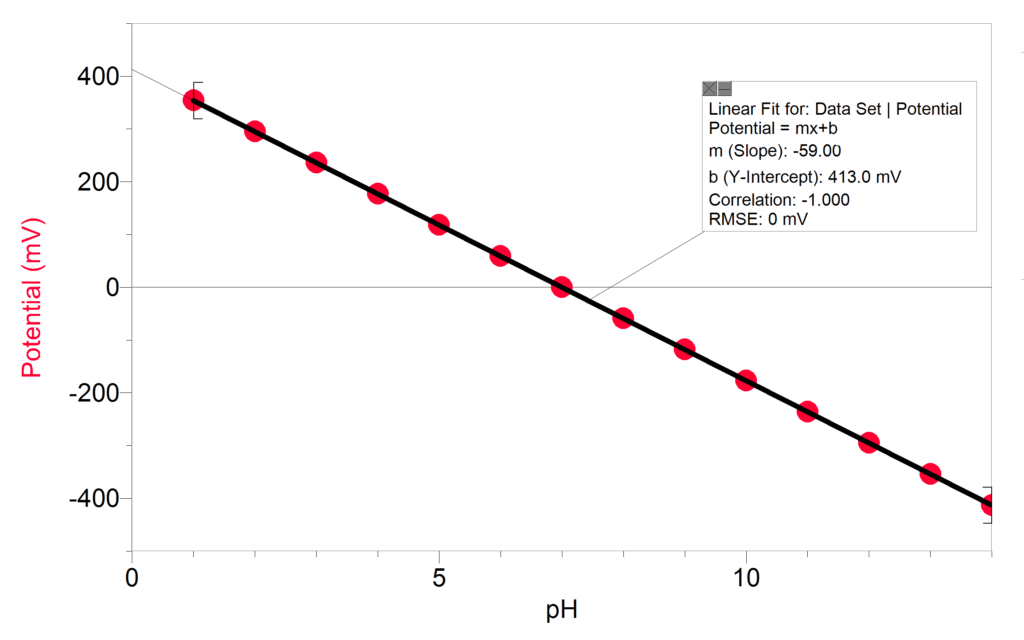
How are pH values measured? pH is a potentiometric measurement where an electrical signal is converted to a pH reading. The signal produced and measured is a potential difference between the sensing and reference electrodes. The theoretical potential at pH 7 is 0 mV and the slope of the line is ~59 mV. This means that, in theory, the pH sensor will change its output by 59 mV for every change in a pH unit. The relationship between the potential and hydrogen ion activity in the sample is described by the Nernst equation.
E = Eo – 2.3 (RT/nF) log aH+
where: E = total potential (in mV) developed between the sensing and reference electrodes
- Eo = standard potential of the electrode at aH+ = 1 mol/L
- R = gas constant
- T = temperature in K
- n = number of electrons
- F = Faraday constant
- aH+ = activity of the hydrogen ion in solution
The term 2.3RT/nF is referred to as the Nernst slope. For an ideal electrode the slope at 25°C is 59.16 mV per decade change in hydrogen ion activity. In reality, the behavior is slightly different than in theory. Calibrating the sensor compensates for this by determining the actual slope and offset using buffers and updating the data-collection software accordingly.
Vernier pH Sensors are combination electrodes. This means the sensor contains both the reference and measuring electrode in one body. When the sensor is placed in the solution, the glass bulb senses the hydrogen ions and the internal electrolyte solution picks up the signal from the glass bulb. The silver/silver chloride/ (Ag/AgCl) reference electrode containing electrolyte generates a constant potential. The difference between the reference and measuring electrodes is a function of the pH value of a solution.
