Introduction
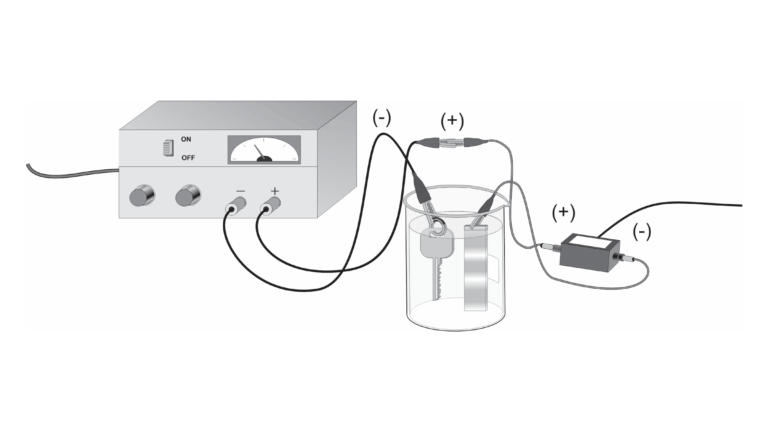
In this experiment, you will conduct, observe, and measure the process of electroplating. This process is used to deposit a layer of metal, such as chromium, copper, or gold, onto another metal. As a commercial process, electroplated coatings are used to improve appearance, resist corrosion, or improve hardness of metallic surfaces. This experiment describes one method of producing a copper coating on a brass key or other suitable metallic object.
You will prepare an electrochemical cell by using a copper strip as the cathode (positive terminal) and a brass key as the anode (negative terminal). The electrodes are immersed in a solution containing acidified copper (II) sulfate. As you apply a potential to the electrodes, you will be effectively transferring Cu atoms from the anode to the surface of the brass key.
In this experiment, you will use one application of Faraday’s law, stated in equation form below.
I is the current in amperes; t is the time that the current is applied, in seconds; MM is the molar mass of the element that is deposited; n is the number of moles of electrons/mol; and 96,500 is ₣, the Faraday constant.
Objectives
In this experiment, you will
- Prepare and operate an electrochemical cell to plate copper onto a brass surface.
- Measure the amount of copper that was deposited in the electroplating process.
- Calculate the amount of energy used to complete the electroplating process.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 2

Option 3

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 3.2.5—Oxidation occurs at the anode and reduction occurs at the cathode in electrochemical cells.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #21 of Advanced Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


